New Study Reveals Genetic Secrets Behind the Rapid Spread of Invasive Malaria Vector
By: TAMU Biology
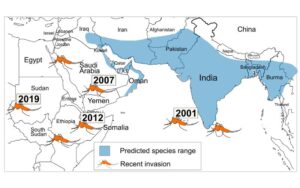 A groundbreaking study led by Dr. Mahul Chakraborty & first author Alex Samano at Texas A&M University Department of Biology reveals how genome structural variants (SVs)—large-scale changes in the genetic code—may be driving the global spread and insecticide resistance of Anopheles stephensi, a dangerous urban malaria vector.
A groundbreaking study led by Dr. Mahul Chakraborty & first author Alex Samano at Texas A&M University Department of Biology reveals how genome structural variants (SVs)—large-scale changes in the genetic code—may be driving the global spread and insecticide resistance of Anopheles stephensi, a dangerous urban malaria vector.
The research paper, published in Molecular Biology and Evolution, analyzed the genomes of 115 An. stephensi mosquitoes collected from both their ancestral range in mainland India and from invasive populations on islands. The team identified nearly 19,000 structural variants (gene duplications and deletions) that may help explain how this species adapts so successfully to new environments.

Alex Samano
While many SVs are likely harmful, a subset show signs of positive selection, suggesting they play a role in the mosquito’s evolutionary success. Notably, the study uncovered three novel gene duplications linked to insecticide resistance, which appear to have evolved independently in different populations. The team also identified candidate mutations associated with tolerance to brackish water in larval stages, a key adaptation in coastal and island regions.

Dr. Mahul Chakraborty
“We found that some of the same mutations linked to insecticide resistance have evolved multiple times in different populations, a strong sign of rapid, ongoing adaptation,” said Dr. Chakraborty. “This highlights how structural variation in the genome. not just single DNA changes. can shape evolutionary success.”
The study found that nearly all of the adaptive SVs in invasive island populations originated from the mainland, showing how existing genetic variation can fuel adaptation and invasion. As urban malaria continues to rise, these findings provide critical insights into the genetic mechanisms behind mosquito invasiveness and resistance to control measures.
“Knowing which mutations are driving success in these mosquitoes gives us new targets for surveillance and potentially for disrupting their spread,” Chakraborty said. This work demonstrates the power of genome-scale research to illuminate the evolutionary dynamics of invasive disease vectors and pests, and may help guide smarter strategies to combat them.





