Tissue Size Regulation
A simple mechanism to sense the number N of type x cells in a tissue or organism would be to have these cells secrete a specific factor X. If each type x cell secretes X at a rate of Φ molecules per second, the average lifetime of the X molecules is T seconds, and the tissue or body volume is V, then the concentration of X will be NΦT/V. This means that there would be a roughly linear relationship between the number of type x cells and the concentration of X.
If the X factor represses proliferation of the x cells when the concentration of X is above a certain concentration, this would then regulate the number of x cells for a given total body volume. If the body volume increased or decreased, the number of x cells would change accordingly to keep the number of x cells a fixed percentage of the body volume. A key requirement of this mechanism is that the X factor is unstable. If there is a decrease (by wounding, for instance) of the number of x cells, the remaining cells will sense their absence as a decrease in X serum concentration within time T.
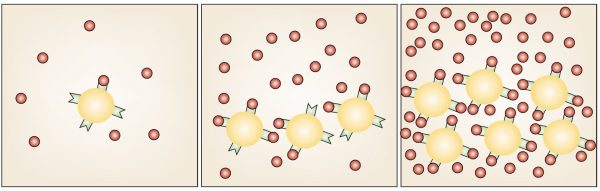
In the figure, a factor (red dots) secreted by the cells (yellow) binds to receptors (green). When there is the correct number of cells, a high concentration of the factor saturates the receptors, causing proliferation to stop.
One way to regulate the size of a group of cells or tissue is to have an autocrine secreted factor inhibit cell proliferation as part of a negative feedback loop. Starting in the 1930’s, a variety of experiments strongly indicated the existence of factors called chalones secreted by cells of a specific tissue that, when they reach a sufficiently high concentration in the blood, inhibit the proliferation of cells of that tissue to regulate tissue size. With the exception of myostatin, a chalone used by muscle cells, the other factors and their signal transduction pathways have eluded identification, with many purification attempts failing. We found that a Dictyostelium secreted protein we named AprA is a Dictyostelium chalone. While examining colonies of cells lacking AprA, we noticed that AprA also acts a chemorepellent. In current work, we found that a second Dictyostelium chalone is polyphosphate (~10-mer linear polymers of phosphate). We are currently using techniques such as shotgun antisense to find signal transduction components that allow cells to sense AprA and polyphosphate. Since the identity of endogenous signals that specifically regulate the size of the liver, or some other tissue, could be useful in a therapeutic setting, we hope that our work on chalones in Dictyostelium will teach us how to successfully revisit the mammalian chalone problem.
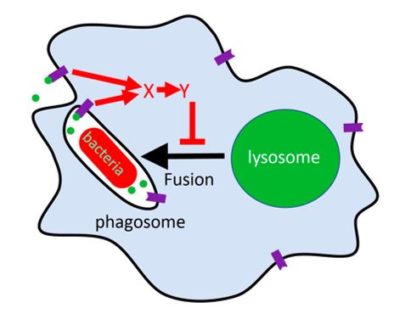
Polyphosphate (green dots) outside cells (Dictyostelium or human macrophages), or secreted by bacteria such as Mycobacterium tuberculosis (red bacteria), binds to polyphosphate receptors (purple) on the cell membrane or on the inside of an endosome, and this activates a signal transduction pathway (red arrows) that blocks the fusion of the bacteria-containing endosome with a lysosome (green). Pharmacologically blocking the red-arrow signal transduction pathway appears to make the cells ignore the polyphosphate signal, and the lysosome fuses with the endosome, killing the bacteria. The pharmacological inhibitors of the red-arrow pathway are thus potential therapeutics for tuberculosis.
Potential additional therapeutics for tuberculosis
Like macrophages, Dictyostelium cells phagocytose and kill bacteria. We noticed that lower concentrations of extracellular polyphosphate cause some Dictyostelium cells to ingest but not kill bacteria, possibly to carry a food supply, much like the farming observed by Debby Brock and Joan Strassman. Mycobacterium tuberculosis (M. tb), the causative bacteria for tuberculosis, is ingested but not killed by macrophages. We found that M. tb also secretes polyphosphate, and that this appears to at least in part cause macrophages to not kill them. Using both Dictyostelium and human macrophages, we are studying how the M. tb polyphosphate signal is sensed by cells, and identifying ways to block this sensing pathway so that macrophages will ignore the polyphosphate ‘don’t kill me’ signal, and kill ingested M. tb. We found that a drug which blocks a polyphosphate signal transduction pathway found in Dictyostelium causes human macrophages to increase their killing of ingested M. tb. Current lab efforts for this project are on expanding understanding of the polyphosphate ‘don’t kill me’ signal transduction pathway to develop more potential therapeutics for tuberculosis.
Key papers
Brock, D.A. and Gomer, R.H. (2005). A secreted factor represses cell proliferation in Dictyostelium. Development, 132, 4553-4562.
Bakthavatsalam, D., Brock, D.A., Nikravan, N.N., Houston, K.D., Hatton, R.D. and Gomer, R.H. The secreted Dictyostelium protein CfaD is a chalone. J. Cell Science, 121, 2473-2480 (2008).
Tang, Y. and Gomer, R.H. A protein with similarity to PTEN regulates aggregation territory size by decreasing cAMP pulse size during Dictyostelium discoideum development. Eukaryotic Cell, 7, 1758-1770 (2008).
Tang, Y. and Gomer, R.H. CnrN regulates Dictyostelium group size using a counting factor-independent mechanism. Communicative & Integrative Biology, 1, 185-187 (2008).
Choe, J. M., Bakthavatsalam, D., Phillips, J.E., and Gomer, R. H. Dictyostelium cells bind a secreted autocrine factor that represses cell proliferation. BMC Biochemistry, 10, 4 (2009).
Bakthavatsalam, D., Choe, J.M., Hanson, N.E. and Gomer, R.H. A Dictyostelium chalone uses G proteins to regulate proliferation. BMC Biology, 7, 44 (2009).
Phillips, J.E. and Gomer, R.H. The ROCO kinase QkgA is necessary for proliferation inhibition by autocrine signals in Dictyostelium discoideum. Eukaryotic Cell, 9, 1557-1565 (2010).
Gomer, R.H., Jang, W., and Brazill, D. Cell density sensing and size determination. Development, Growth & Differentiation, 53,482-494 (2011).
Phillips, J.E., Huang, E., Shaulsky, G., and Gomer, R.H. The Putative bZIP transcription factor BzpN slows proliferation and functions in the regulation of cell density by autocrine signals in Dictyostelium. PLoS ONE, 6, e21765 (2011).
Herlihy, S.E., Tang, Y., and Gomer, R.H. A Dictyostelium secreted factor requires a PTEN-like phosphatase to slow proliferation and induce chemorepulsion. PLoS ONE, 8, e59365 (2013).
Bakthavatsalam, D., White, M.J.V., Herlihy, S.E., Phillips, J.E., and Gomer, R.H. A Retinoblastoma orthologue is required for the sensing of a chalone in Dictyostelium. Eukaryotic Cell, 13, 376-382 (2014).
Phillips, J.E. and Gomer, R.H. The p21-activated kinase (PAK) family member PakD is required for chemorepulsion and proliferation inhibition by autocrine signals in Dictyostelium discoideum. PLoS ONE, 9, e96633 (2014).
Suess, P.M. and Gomer, R.H. Extracellular polyphosphate inhibits proliferation in an autocrine negative feedback loop in Dictyostelium discoideum. Journal of Biological Chemistry, 291, 20260-9 (2016).
Herlihy, S.E., Tang, Y., Phillips, J.E., and Gomer, R.H. Functional similarities between the Dictyostelium protein AprA and the human protein Dipeptidyl-Peptidase IV. Protein Science, 26, 578-585 (2017).
Suess, P.M., Watson, J., Chen, W., and Gomer, R.H. Extracellular polyphosphate signals through Ras and Akt to prime Dictyostelium discoideum cells for development. J. Cell Science, 130, 2394-2404 (2017).
Tang, Y., Wu, Y., Herlihy, S.E., Brito-Aleman, F.J., Ting, J.H., Janetopoulos, C., and Gomer, R.H. An autocrine proliferation repressor regulates Dictyostelium discoideum proliferation and chemorepulsion using the G protein-coupled receptor GrlH. mBio, 9, e02443-17 (2018).
Suess, P.M., Tang, Y., and Gomer, R.H. The putative G protein-coupled receptor GrlD mediates extracellular polyphosphate sensing in Dictyostelium discoideum. Molecular Biology of the Cell, 30, 1118-1128 (2019).
Suess, P.M., Chinea, L.E., Pilling, D., and Gomer, R.H. Extracellular polyphosphate promotes macrophage and fibrocyte differentiation, inhibits leukocyte proliferation, and acts as a chemotactic agent for neutrophils. Journal of Immunology, 203, 493-499 (2019).
Gomer, R.H. The use of diffusion calculations and Monte Carlo simulations to understand the behavior of cells in Dictyostelium communities. Computational and Structural Biotechnology Journal, 17, 684-688 (2019).
Rijal, R., Cadena, L.A., Smith, M.R., Carr, J.F., and Gomer, R.H. Polyphosphate is an extracellular signal that can facilitate bacterial survival in eukaryotic cells. Proc. Natl. Acad. Sci. USA, 117, 31923-31934 (2020).
Tang, Y., Zimmerhanzel, D.E., McCullough, J.R., Cadena, L.A., and Gomer, R.H. An Autocrine Negative Feedback Loop Inhibits D. discoideum Proliferation Through an IP3/ Ca2+ Pathway. mBio, 12, e0134721 (2021).
